
Risk Evaluation and Mitigation Strategy (REMS)
|
|
|
|
Counseling for Patients of Reproductive Potential*Qsymia can cause fetal harm. Advise patients of reproductive potential that labeling recommends:
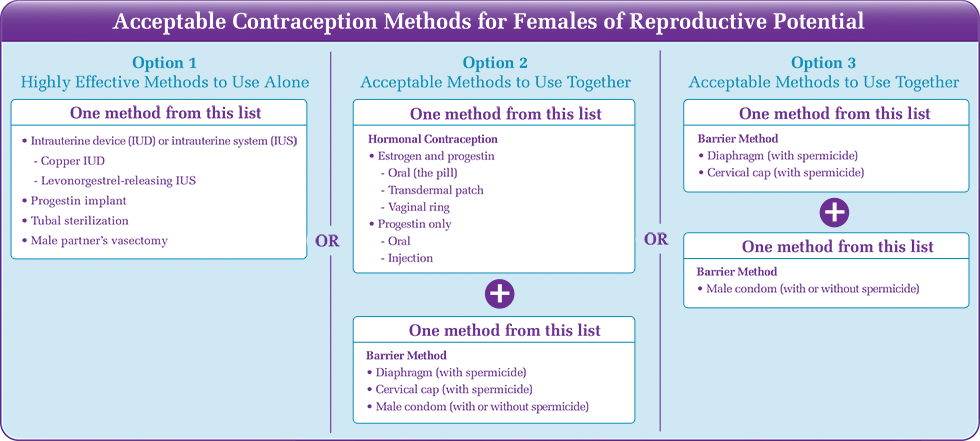 *Patients of reproductive potential are patients who have NOT had a hysterectomy, bilateral oophorectomy, or medically documented spontaneous ovarian failure, and have not gone through menopause. Menopause should be clinically confirmed by an individual's healthcare provider. Advise nursing mothers not to use Qsymia. Qsymia may be present in human milk because topiramate and amphetamines (phentermine has pharmacologic activity and a chemical structure similar to amphetamines) are excreted in human milk. |
